Microneedle Flu Vaccine Market Introduction and Overview
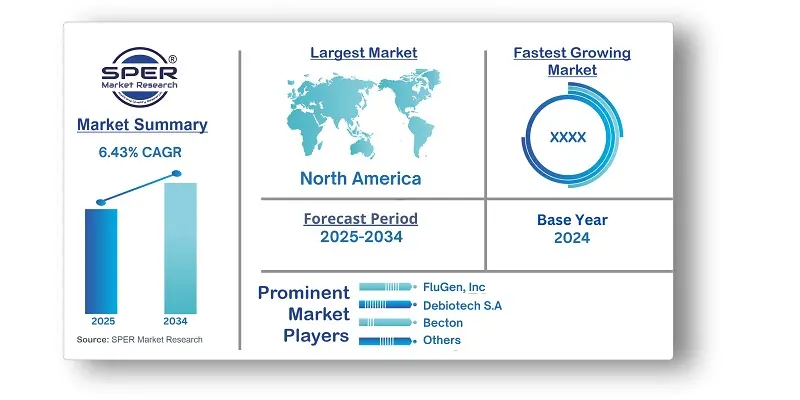
According to SPER Market Research, the Global Microneedle Flu Vaccine Market is estimated to reach USD 2.96 billion by 2034 with a CAGR of 6.43%.
The report includes an in-depth analysis of the Global Microneedle Flu Vaccine Market, including market size and trends, product mix, Applications, and supplier analysis. The microneedle flu vaccine market is expected to increase significantly as the global influenza incidence rises and important industry players in the field of microneedle flu vaccines collaborate on R&D activities. Furthermore, patient convenience and acceptance are key market drivers since they remove a significant barrier to traditional needle-based vaccines. Microneedle patches are intended to accurately transfer medications into the intradermal area, which is rich in immune cells, and to provide a non-invasive and self-applicable vaccination method, eliminating the need for hypodermic needles and skilled medical staff for vaccine administration. However, environmental factors such as skin hydration may alter drug delivery, and the high cost of microneedle vaccinations in comparison to standard vaccines may limit market growth throughout the projection period.
By Product Type Insights: The microneedle flu vaccine market is divided into two products, solid microneedle, and hollow microneedle. The solid microneedle product dominates, as patients find them extremely acceptable because they are painless and minimally intrusive, leading in increased immunization compliance and convenience. Solid microneedles can deliver vaccinations directly into the skins dermal layer, which contains a large number of immune cells. This tailored administration can boost the immunological response to the vaccination, potentially increasing vaccine efficacy compared to typical intramuscular injections.
By Vaccine Type Insights: In the Microneedle Flu Vaccine market, the vaccine types are trivalent flu vaccine, Quadrivalent flu vaccine. The trivalent flu vaccine category is dominant, as it defends against three different flu virus strains (usually two influenza A strains and one influenza B strain), make up a sizable percentage of the annual flu vaccination market. Companies that develop microneedle delivery technologies exclusively for trivalent flu vaccinations can obtain a competitive advantage in an established market niche.
By Region Insights: North America, specifically the Microneedle Flu Vaccine Market, dominated the market in 2024, owing to the region's well-developed and advanced healthcare infrastructure, which creates an ideal environment for the development, manufacturing, and distribution of microneedle-based flu vaccines. The regions strong healthcare system promotes research, clinical trials, and regulatory clearances, promoting market expansion.
Market Competitive Landscape:
The competitive landscape of the microneedle flu vaccine industry is marked by the existence of several important companies vying for a sizable market share. These companies are focussing on strategic alliances, collaborations, and acquisitions to strengthen their product portfolios and market presence. In addition, new firms are entering the market, increasing competition. With the increasing demand for painless and convenient vaccination methods, the industry is projected to become more competitive and innovative in the future years.
Recent Developments:
- November 2022: Micron Biomedical, Inc. has raised USD 14 million in Series A funding to assist the company's commercial production development and establish a strong cooperation with LTS Lohmann. Micron's development pipeline includes vaccines and drugs in collaboration with pharmaceutical companies, foundations, and government agencies.
- April 2022: Vaxess Technologies, Inc., an innovative life sciences business creating a pipeline of shelf-stable and easy-to-apply vaccinations, has announced the manufacturing of the first GMP batch of MIMIX Technology Vaccine Patches for an upcoming Phase I seasonal influenza clinical study.
Scope of the Report:
| Report Metric | Details |
| Market size available for years | 2021-2034 |
| Base year considered | 2024 |
| Forecast period | 2025-2034 |
| Segments covered | By Product Type, Vaccine Type. |
| Regions covered | North America, Asia-Pacific, Latin America, Middle East & Africa and Europe. |
| Companies Covered | Becton, CosMED Pharmaceuticals Co., Ltd, Debiotech S.A, Dickinson and Company, FluGen, Inc, MERCK & CO., INC, Microdermics Inc, NanoPass Technologies Limited, PFIZER, INC, TSRL Inc, Vaxess Technologies. and others. |
Key Topics Covered in the Report:
- Global Microneedle Flu Vaccine Market Size (FY’2021-FY’2034)
- Overview of Global Microneedle Flu Vaccine Market
- Segmentation of Global Microneedle Flu Vaccine Market By Product Type (Solid Microneedle and Hollow Microneedle)
- Segmentation of Global Microneedle Flu Vaccine Market, By Vaccine Type (Trivalent Flu Vaccine, Quadrivalent Flu Vaccine)
- Statistical Snap of Global Microneedle Flu Vaccine Market
- Expansion Analysis of Global Microneedle Flu Vaccine Market
- Problems and Obstacles in Global Microneedle Flu Vaccine Market
- Competitive Landscape in the Global Microneedle Flu Vaccine Market
- Details on Current Investment in Global Microneedle Flu Vaccine Market
- Competitive Analysis of Global Microneedle Flu Market
- Prominent Players in the Global Microneedle Flu Vaccine Market
- SWOT Analysis of Global Microneedle Flu Market
- Global Microneedle Flu Market Future Outlook and Projections (FY’2025-FY’2034)
- Recommendations from Analyst
1. Introduction
1.1. Scope of the report
1.2. Market segment analysis
2. Research Methodology
2.1. Research data source
2.1.1. Secondary Data
2.1.2. Primary Data
2.1.3. SPERs internal database
2.1.4. Premium insight from KOLs
2.2. Market size estimation
2.2.1. Top-down and Bottom-up approach
2.3. Data triangulation
3. Executive Summary
4. Market Dynamics
4.1. Driver, Restraint, Opportunity and Challenges analysis
4.1.1. Drivers
4.1.2. Restraints
4.1.3. Opportunities
4.1.4. Challenges
5. Market variable and outlook
5.1. SWOT Analysis
5.1.1. Strengths
5.1.2. Weaknesses
5.1.3. Opportunities
5.1.4. Threats
5.2. PESTEL Analysis
5.2.1. Political Landscape
5.2.2. Economic Landscape
5.2.3. Social Landscape
5.2.4. Technological Landscape
5.2.5. Environmental Landscape
5.2.6. Legal Landscape
5.3. PORTERs Five Forces
5.3.1. Bargaining power of suppliers
5.3.2. Bargaining power of buyers
5.3.3. Threat of Substitute
5.3.4. Threat of new entrant
5.3.5. Competitive rivalry
5.4. Heat Map Analysis
6. Competitive Landscape
6.1. Global Microneedle Flu Vaccine Market Manufacturing Base Distribution, Sales Area, Product Type
6.2. Mergers & Acquisitions, Partnerships, Product Launch, and Collaboration in Global Microneedle Flu Vaccine Market
7. Global Microneedle Flu Vaccine Market, By Product Type (USD Million) 2021-2034
7.1. Solid Microneedle
7.2. Hollow Microneedle
8. Global Microneedle Flu Vaccine Market, By Vaccine Type (USD Million) 2021-2034
8.1. Trivalent Flu Vaccine
8.2. Quadrivalent Flu Vaccine
9. Global Microneedle Flu Vaccine Market, (USD Million) 2021-2034
9.1. Global Microneedle Flu Vaccine Market Size and Market Share
10. Global Microneedle Flu Vaccine Market, By Region, (USD Million) 2021-2034
10.1. Asia-Pacific
10.1.1. Australia
10.1.2. China
10.1.3. India
10.1.4. Japan
10.1.5. South Korea
10.1.6. Rest of Asia-Pacific
10.2. Europe
10.2.1. France
10.2.2. Germany
10.2.3. Italy
10.2.4. Spain
10.2.5. United Kingdom
10.2.6. Rest of Europe
10.3. Middle East and Africa
10.3.1. Kingdom of Saudi Arabia
10.3.2. United Arab Emirates
10.3.3. Qatar
10.3.4. South Africa
10.3.5. Egypt
10.3.6. Morocco
10.3.7. Nigeria
10.3.8. Rest of Middle-East and Africa
10.4. North America
10.4.1. Canada
10.4.2. Mexico
10.4.3. United States
10.5. Latin America
10.5.1. Argentina
10.5.2. Brazil
10.5.3. Rest of Latin America
11. Company Profile
11.1. Becton
11.1.1. Company details
11.1.2. Financial outlook
11.1.3. Product summary
11.1.4. Recent developments
11.2. CosMED Pharmaceuticals Co., Ltd
11.2.1. Company details
11.2.2. Financial outlook
11.2.3. Product summary
11.2.4. Recent developments
11.3. Debiotech S.A
11.3.1. Company details
11.3.2. Financial outlook
11.3.3. Product summary
11.3.4. Recent developments
11.4. Dickinson and Company
11.4.1. Company details
11.4.2. Financial outlook
11.4.3. Product summary
11.4.4. Recent developments
11.5. FluGen, Inc
11.5.1. Company details
11.5.2. Financial outlook
11.5.3. Product summary
11.5.4. Recent developments
11.6. MERCK & CO., INC
11.6.1. Company details
11.6.2. Financial outlook
11.6.3. Product summary
11.6.4. Recent developments
11.7. Microdermics Inc
11.7.1. Company details
11.7.2. Financial outlook
11.7.3. Product summary
11.7.4. Recent developments
11.8. NanoPass Technologies Limited
11.8.1. Company details
11.8.2. Financial outlook
11.8.3. Product summary
11.8.4. Recent developments
11.9. PFIZER, INC
11.9.1. Company details
11.9.2. Financial outlook
11.9.3. Product summary
11.9.4. Recent developments
11.10. TSRL Inc
11.10.1. Company details
11.10.2. Financial outlook
11.10.3. Product summary
11.10.4. Recent developments
11.11. Vaxess Technologies
11.11.1. Company details
11.11.2. Financial outlook
11.11.3. Product summary
11.11.4. Recent developments
11.12. Others
12. Conclusion
13. List of Abbreviations
14. Reference Links
SPER Market Research’s methodology uses great emphasis on primary research to ensure that the market intelligence insights are up to date, reliable and accurate. Primary interviews are done with players involved in each phase of a supply chain to analyze the market forecasting. The secondary research method is used to help you fully understand how the future markets and the spending patterns look likes.
The report is based on in-depth qualitative and quantitative analysis of the Product Market. The quantitative analysis involves the application of various projection and sampling techniques. The qualitative analysis involves primary interviews, surveys, and vendor briefings. The data gathered as a result of these processes are validated through experts opinion. Our research methodology entails an ideal mixture of primary and secondary initiatives.